Molecular Compounds Dissolve In Water To Form Ions Is
To allow chemists to. In the complete ionic equation soluble ionic compounds and strong acids are rewritten as dissociated ions.

1 16 Do Now List 3 Ways That Protons And Electrons Are Different Ppt Download
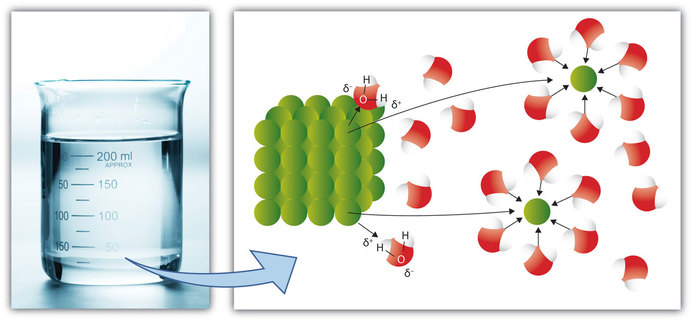
For example ions such as table salt NaCl dissolve easily in water because the positive ions are drawn to the negative oxygen and negative ions to positve hydrogens.

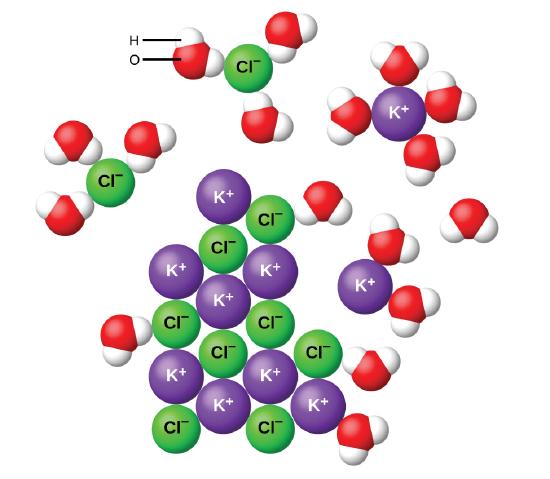
Molecular compounds dissolve in water to form ions is. A phospholipid is a type of lipid molecule that is the main component of the cell membraneLipids are molecules that include fats waxes and some vitamins among others. When CaNO 3 2 dissolves in water Figure 710 the Ca2 ions separate from the NO 3 ions with the oxygen ends of water molecules surrounding the calcium ions and the hydrogen ends of water molecules surrounding the nitrate ions. Solvation or dissolution describes the interaction of solvent with dissolved molecules.
35 Naming Ions and Ionic Compounds. Also the common name is usually not recognized internationally. Covalent compounds can be either polar or nonpolar but they contain weaker bonds than ionic compounds because they.
In the molecular equation for a reaction all of the reactants and products are represented as neutral molecules even soluble ionic compounds and strong acids. The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways. They exist in the form of molecules surrounded by water molecules.
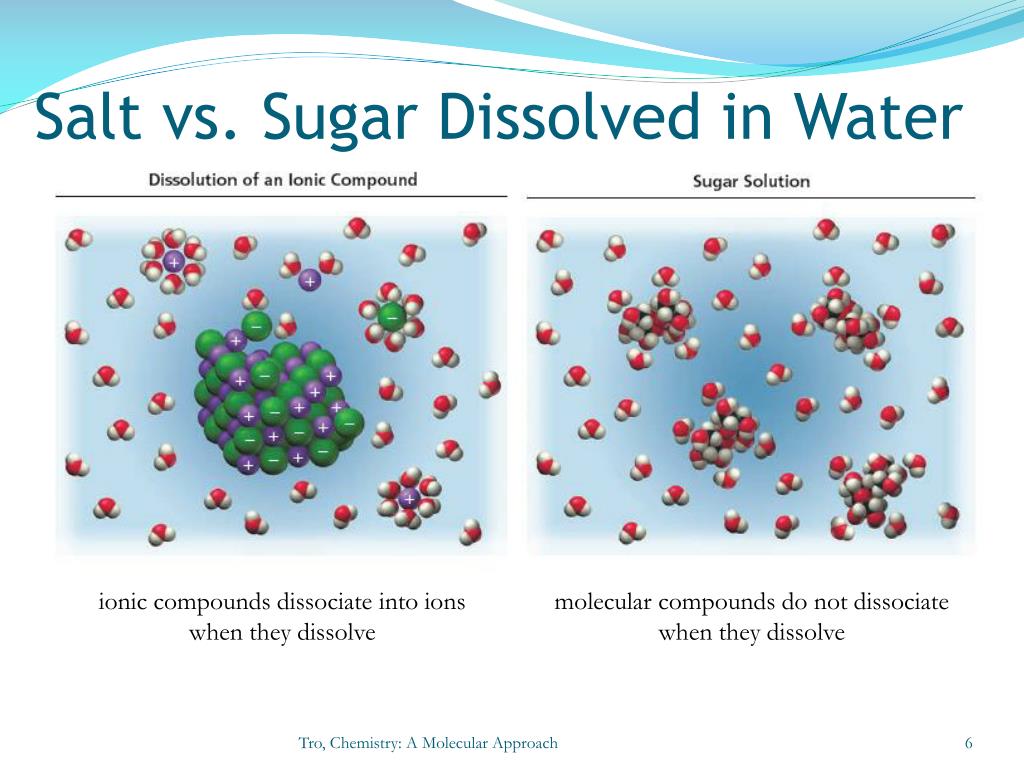
Both ionized and uncharged molecules interact strongly with solvent and the strength and nature of this interaction influence many properties of the solute including solubility reactivity and color as well as influencing the properties of the solvent such as the viscosity and density. This is because covalent molecular compounds cannot form ions when dissolved in water. Many covalent molecular compounds do not dissolve in water.
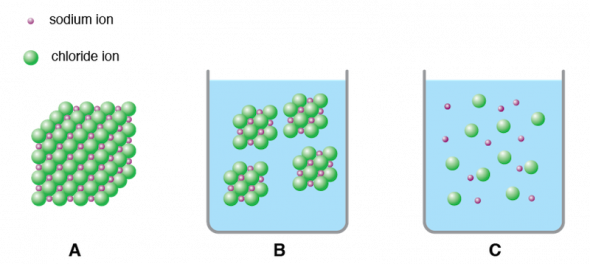
This module explores two common types of chemical bonds. When ionic compounds dissolve the ions separate and become surrounded by water molecules. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
However when a covalent compound is dissolved in water the solution cannot conduct electricity. In the net ionic equation any ions that do not participate in the reaction called spectator ions are excluded. Ionic compounds dissolve in polar solvents like water stack neatly on each other to form crystals and require a lot of energy for their chemical bonds to break.
But there are exceptions as well. What looks like water to you might look like agua or vatten to someone else. Oxygen has a high electronegativity or electron-loving making the oxygen end of a water molecule slightly negative and the hyrogen end slightly positive.
The module presents chemical bonding on a sliding scale from pure covalent to pure ionic depending on differences in the electronegativity of the bonding atoms. 1 oxides containing oxide ions O 2 2 peroxides containing peroxide ions O 2 2 which contain oxygen-oxygen covalent single bonds and 3 superoxides containing superoxide ions O 2 which also have oxygen-oxygen covalent bonds but with one fewer negative charge than peroxide. When many phospholipids line up they form a double layer that is characteristic of all cell.
Water is the chemical substance with chemical formula H 2 O. Basically any material with a composition that includes more than one element symbol or that has a subscript following an element symbol is a molecule or compound rather than an atom. The alkali metals and alkaline earth metals form three different types of binary oxygen compounds.
This can easily be observed in a water-filled bath or. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Each phospholipid is made up of two fatty acids a phosphate group and a glycerol molecule.
Examples of substances that are not atoms include water H 2 O table salt NaCl and ozone O 3. However there are thousands of other compounds that are uncommon or have multiple names. Some compounds have common names like water for H 2 O.
These nonpolar molecular solids will not dissolve in water but will dissolve in a. Ionic compounds form crystals that are composed of oppositely.

Solved What Happens When Ionic Compounds Dissolve In Water Chegg Com
9 3 The Dissolution Process Chemistry Libretexts

Chapter 5 Molecular View Of Reactions In Aqueous Solutions Ppt Download

Dissolution In Water A When An Ionic Co Clutch Prep

General Solution Chemistry Molarity Properties Of Solutions 1
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora

How Do Aqueous Solutions Of Ionic Molecular Compounds Differ Study Com

Chemistry 101 Chap 4 Aqueous Reactions And Solution Stoichiometry 1 General Properties Of Aqueous Solutions 2 Precipitation Reactions 3 Acid Base Ppt Download

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Chapter 4 Reactions In Aqueous Solution Part 1 Of 8 Youtube
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts

Sort The Following Compounds Based On Whet Clutch Prep

Dissolving Process Chemistry For Non Majors
What Happens When Ionic Compounds Dissolve In Water Quora
Ppt Chapter 4 Powerpoint Presentation Free Download Id 762021
Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth
