Five Examples Of Polar Covalent Bond
Note that each of these molecules has a nonpolar hydrocarbon chain the tail and a polar often ionic head group. On non-polar surfaces the adhesive mechanisms may include van der Waals forces whereas on polar surfaces mechanisms such as hydrogen bonding and binding to or forming bridges via.
0 1 Chemistry The Building Blocks Of Molecules Gpc By Openstax Page 5 29 Jobilize
This results in a filled outermost shell.
Five examples of polar covalent bond. Water - A Polar Molecule In this video Paul Andersen explains how the polarity of water makes life on the planet possible. Many permanent bioadhesives eg the oothecal foam of the mantis are generated by a mix to activate process that involves hardening via covalent cross-linking. The enthalpy of a given molecular interaction between two non-bonded atoms is 1 - 10 kcalmole 4 - 42 kjoulemole which in the lower limit is on the order of RT and in the upper limit is significantly less than a covalent bond.
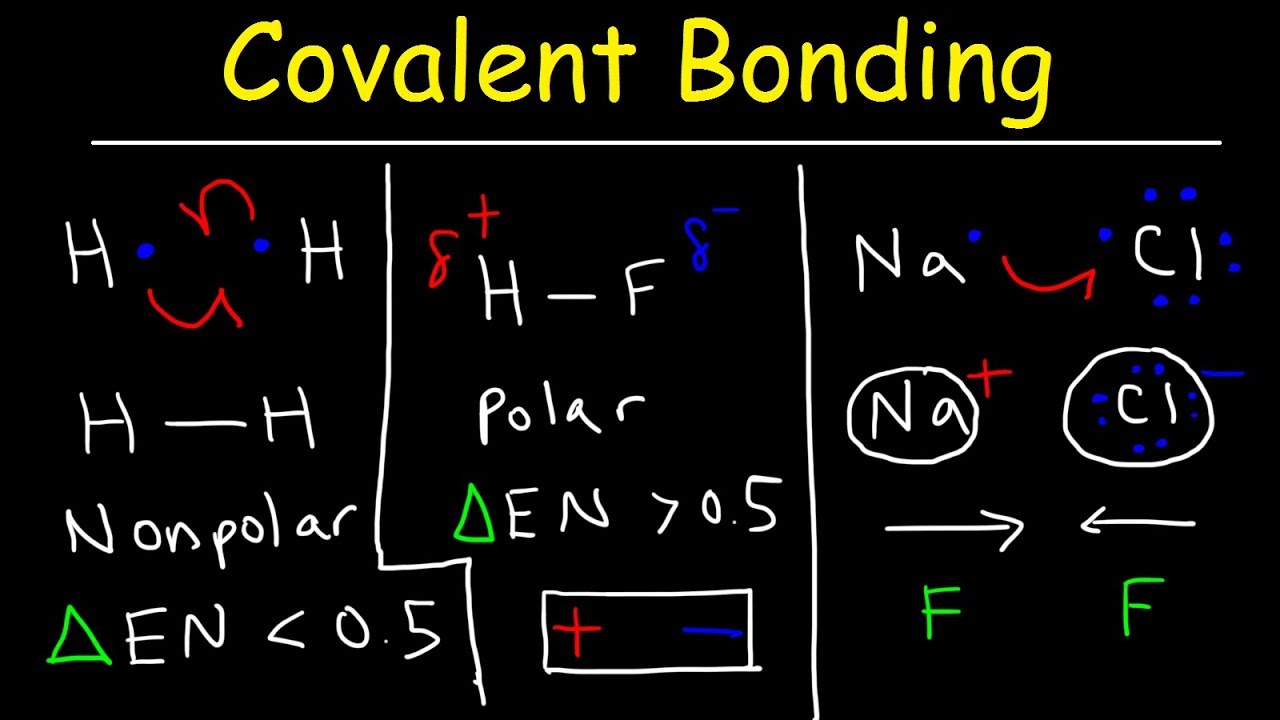
Electronegativity is probably the most important concept to understand in organic chemistry were going to use the definition that Linus Pauling gives in his book the nature of the chemical bond so Linus Pauling says that electronegativity refers to the power of an atom in a molecule to attract electrons to itself so if I look at a molecule Im going to compare two atoms in that molecule Im. Molecules with polar covalent bonds can have a dipole moment an asymmetrical distribution of charge that results in a tendency for molecules to align themselves in an applied electric field. I Ribose ii Glucose iii Maltose iv Lactose v Galactose.
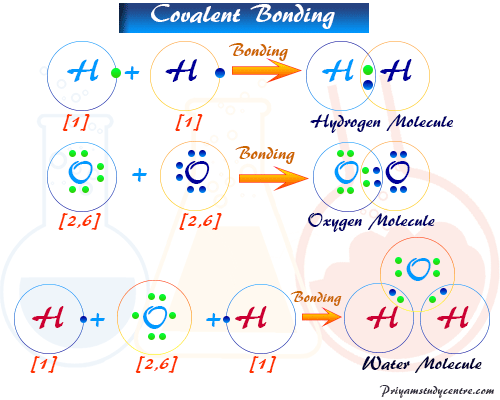
The methane molecule provides an example. Diatomic molecules are molecules composed of only two atoms of the same or different chemical elementsThe prefix di-is of Greek origin meaning two. If a diatomic molecule consists of two atoms of the same element such as hydrogen H 2 or oxygen O 2 then it is said to be homonuclearOtherwise if a diatomic molecule consists of two different atoms such as carbon monoxide CO or.
Any diatomic molecule with a polar covalent bond has a dipole moment but in polyatomic molecules the presence or absence of a net dipole moment depends. The most common examples of such compounds are soaps and detergents four of which are shown below. Therefore carbon atoms can form four covalent bonds with other atoms to satisfy the octet rule.
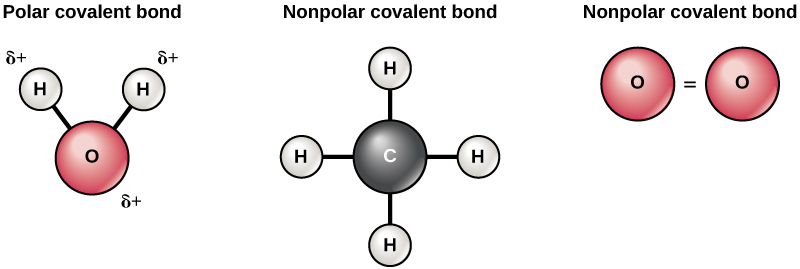
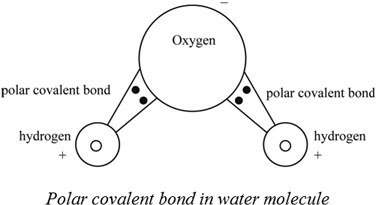
Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. When two bonded atoms have a difference of between 04 and 20 electronegativity units see Table 2 the electrons are shared unequally and the bond is a polar covalent bond there is an unsymmetrical distribution of electrons between the bonded atoms because one atom in the bond is pulling on the shared electrons harder than the. The bonding electrons in polar covalent bonds are not shared equally and a bond moment results.
Following are the five examples of reducing sugars. For example tetrachloro-methane carbon tetrachloride CCl 4 has polar CCl bonds but the tetrahedral arrangement of the four bonds about the central carbon atom causes the individual bond moments to cancel. Bonds hold atoms together within molecules.
Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. It has the chemical formula CH 4. Access detailed answers to various other Science and Maths questions at BYJUS.
This lets us find the most appropriate writer for any type of assignment. However a molecule may be polar or nonpolar depending on its geometry. Oxygen is highly electronegative and pulls the electrons closely creating a partial negative charge.
A molecule is a group of atoms that associates.

Covalent Bonds Biology For Majors I

Difference Between Polar Covalent Bond And Non Polar Covalent Bond Class 11 Chemistry In Urdu Youtube

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples
Covalent Bond Definition Types Polar And Non Polar Covalent Bond Explained

Nonpolar Covalent Bond Definition And Examples

What Is A Polar Covalent Bond Quora

Nonpolar Covalent Bond Definition And Examples

Polar Covalent Bond Definition And Examples
Covalent Bond Types Definition Properties Examples

4 2 Polar And Non Polar Covalent Bonds Sl Youtube

Polar And Non Polar Covalent Compounds Chemical Bonding Cbse Grade 9 Chemistry Youtube
What Is A Polar Covalent Bond Quora

Polar Covalent Bond Definition And Examples

Bond Polarity Chemistry For Non Majors
Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding Types Of Chemical Bonds Youtube
Definition Of Polar Covalent Bond Chegg Com
Difference Between Non Polar And Polar Covalent Bonds Difference Between

Polar Covalent Bond Definition And Examples
